
Cloning popular brands of mineral water is now simpler then ever before with the updated version of the mineral water calculator!
When I blogged about DIY mineral water last year it was mainly a theoretical exercise since I didn’t have the required salts at hand. My experience was limited to adding some baking soda (sodium bicarbonate) to water before carbonation. Luckily Paul Hinrichs tested the calculator! In the meantime I have purchased the required salts and with several kilograms in total I’m probably well stocked for the next decade! Based on the output from the calculator, I mixed the salts required to clone San Pellegrino, added water and carbonated the mixture. And the good news is that it works! The water tastes great and I’ve been enjoying cloned mineral waters every day now for the last couple of weeks.
Some changes have been made to the mineral water calculator (Updated! – scroll down for download options) since I last posted:
- a simpler worksheet more suitable for printing has been added
- more mineral waters have been added to the database, covering TDS (total dissolved solids) levels all the way up to more than 4000 mg/L
- potassium bicarbonate, magnesium chloride and calcium nitrate are made optional and can be left out if desired (it’s still a little unclear to me to what extent these can be detected at the typical levels found in mineral waters)
- one can now chose between using either hydroxides or carbonates of calcium and magnesium, depending on availability (it should be noted however that some waters high in bicarbonate may require the use of the hydroxides – not quite sure about this though)

A spoon full of mineral salts is required for the preparation of 1 liter of San Pellegrino mineral water.
Instructions for how to prepare the mixture of salts
Start by chosing the mineral water you want to clone from the drop down list. My advice would be not to start with the waters having very high levels of total dissolved solids (TDS) (except Kessel and Vichy Saint-Yorre since sodium bicarbonate dissolves easily). Aim for a TDS in the range 200-1500 mg/L (the list of all mineral waters in the rightmost worksheet is viewable and sortable). At the lower end you may not detect much mineral taste at all. At the higher end the mineral taste becomes quite pronounced. You can click the check boxes to include/exclude some salts. If known enter the composition of your tap water (your local water company should be able to give you these figures). I suggest that you weigh out the salts for 10 or even 100 liters, otherwise the amounts of salts will be in the low milligram or microgram range, requiring expensive lab scales. Mix the salts well. It may be god to start by mixing the salts present in the lowest concentrations first to ensure a homogeneous mixture.
How to make a cloned mineral water
Weigh out the approximate amount of salt (prepared as described above) needed for the amount of water that your carbonation vessel holds. At this point it doesn’t need to be very accurate, so if you have weighed it once you can simply need to remember which spoon you used and the size of the heap. Note that the different mineral salts vary greatly in density, so you should calibrate the heap used for each mineral salt mixture. Add the salt to the carbonation vessel and fill it up to the mark with water. The water will now turn opaque and whitish as the salts are suspended in the water (see picture above). Carbonate carefully and, depending on whether the water is high in carbonation and/or bicarbonate, try to hold the carbonation pressure for a couple of seconds extra before letting the pressure out. This allows a little more carbon dioxide to dissolve. Screw on the cap immediately to prevent the carbon dioxide from escaping. In some cases it may be necessary to repeat the carbonation step after some hours. Once the salts have dissolved (i.e. the water becomes clear) you can enjoy your very own home-made mineral water!

Several of the mineral salts have are not soluble in tap water, hence the opaque look to the left. After carbonation however they dissolve rapidly.
So far I’ve made up the salt mixtures for San Pellegrino (total dissolved solids, TDS: 1109 mg/L) and Gerolsteiner (TDS: 2488 mg/L). The first works like a charm, even when all salts are added simultaneously. This is possibly due to the high amount of sulfates which seem to dissolve more easily. Gerolsteiner is more tricky, partly due to the high TDS and the low amount of sulfate. I made it using carbonates instead of hydroxides, hoping that this would require addition of less carbon dioxide to neutralize the base. But after two days and 2-3 extra additions of carbon dioxide the salts had still not dissolved completely and this puzzles me. I certainly need to repeat this experiment. Darcy O’Neil states in Fix the pumps that the order of addition does matter. I’m not quite sure if that really is the case as most of the salts have a very low water solubility to start with, and the carbonic acid is the reason they dissolve. But maybe there is something I’m overlooking here? It could be that Gerolsteiner is easier to do with hydroxides, but Paul Hinrichs also had some trouble getting all the salts to dissolve for Gerolsteiner.
Some of the salts may be tricky to obtain, but the synonyms below may be of some help:
- CaSO4·0.5H2O = Plaster of Paris
- MgSO4·7H2O = Epsom salt
- CaCO3 = Chalk
- NaHCO3 = Baking soda
- NaCl = Table salt
- Mg(OH)2 = Milk of magnesia
- Ca(OH)2 = Slaked lime, pickling lime, CAL
Before you rush to buy these salts I should probably add some words of warning: make sure that the source you find is suitable for consumption! Some technical qualities of mineral salts may not be intended for food use, for instance due to the presence of heavy metals or other contaminants. Note that some of the salts are available with varying amounts of crystal water. If you use other salts than those specified (i.e. anhydrous salts or salts with more crystal water) the molecular weights in the spreadsheet need to be adjusted for this. I guess that if you are familiar with the concept of crystal water, you’ll easily figure out the correct molecular weight and how to update the calculator according to the specific salts you chose to use.
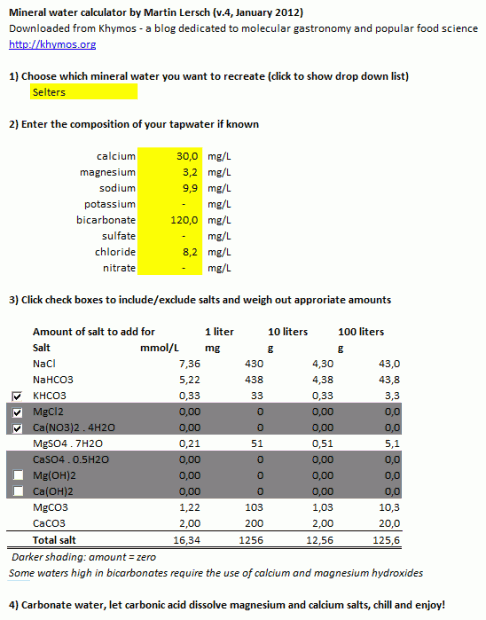
Screen shot of the simple version, best suited for printing (see below for download options):
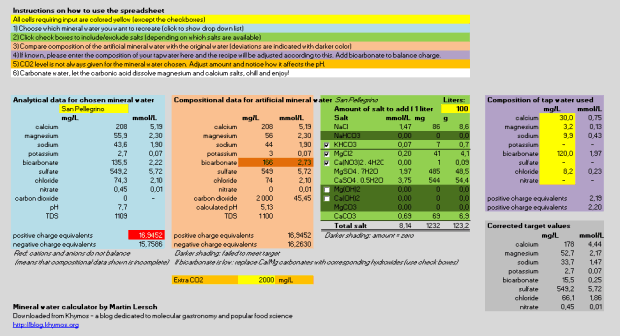
Screen shot of the complete version (see below for download options):
Calculator download options
Version 6 (latest update, January 2015 – some extra waters added)
Excel: mineral_water_calculator_v6.xlsx
Version 5
Excel: mineral_water_calculator_v5.xlsx
Open office: mineral_water_calculator_v5.ods
Version 4 (the version originally provided with this blog post – contains errors)
mineral_water_calculator_v4.xlsx
Mineral waters included
Mineral waters included in the database that comes with the calculator: Acqua Panna, Antipodes, Apollinaris, Aquarel Birken, Artificial mineral water, Badoit, Borsec, Burton (beer brewing), Calistoga, Carola Rouge, Contrex, Dorna, Evian, Farris, Fiuggi, Gerolsteiner, Harghita, Hassia Sprudel, Henniez, Kessel, London (beer brewing), Mountain Valley Spring, Munich (beer brewing), Neuselters, Perrier, Pilsen (beer brewing), PurPur (coffee brewing), Rosbacher Klassich, Saint-Yorre, Salvus, San Benedetto, San Narciso, San Pellegrino, SCAA target (for coffee brewing), Selters, Tea brewing (max), Tea brewing (min), Tesanjski Dijamant, Topo chico, Ty Nant, Vittel, Volvic, Voss, Waiwera. And you can easily add data for other mineral waters. The websites mineralwaters.org, finewaters.com and Mineral water atlas of the world have data for several hundred waters available. And if you have a bottle of your favourite mineral water at hand you only need to check the label to find the required input for the calculator.

Anyway to save it not as xlsx? cant seem to use it on openoffice excel, it comes across as read only cant pick the mineral water
but this is pretty awesome!
Weston: I’m working on it!
Mineral water calculator has now been updated. Version 5 is available for download in Excel as well as Open office format.
Formula for calculating bicarbonate concentration in final water now gives the same result when using hydroxides or carbonates.
Special waters for brewing tea, coffee and beer have been added. Also some obvious errors have been fixed. In a few cases the bicarbonate level has been adjusted to achieve a more or less charge neutral mixture.
The mineral composition of Burton water is quite similar to that of San Pellegrino.
Burton: Ca 268/Mg 62 /Na 30/Sulfate 638/Chloride 36/Nitrate 31
San Pellegrino: Ca 208/Mg 57/Na 45/Sulfate 549/Chloride 74/Nitrate 2
Burton salt is available from beer brewing stores.
How do one account for the mineral contents of tap water if you do not have a lab at home? This will certainly affect the result. Other than that this is ingenious!
Kristian: Contact your local water supplier and ask if they can provide you with an analytical report of the water. I’m quite sure they have the data available.
This sounds like a great idea but it’s really impractical. I assume you would have to source the salts/minerals from multiple suppliers and you would have to buy in bulk. You know what would be cool? If a person bought these raw ingredients and put together small mixtures, packaged in sealed bags or jars and sold it at a profile. You can guy one for San Pellegrino or Perrier. I assume the per cup or per litre cost is extremely small. You could sell them for a huge profit and it would still be attractive/convenient to people like me.
You should include the common names in the table alongside the chemical formulae on the spreadsheet.
David: As mentioned above, the Burton salt available from brewing stores is a good approximation of San Pellegrino.
Nessie: Check the “Calculator” sheet and scroll down. There you find the complete list with common names.
[…] you want in your water. It really helps if you have a favorite brand of mineral water to copy. Martin Lersch of the blog Khymos created a series of Excel spreadsheets that shows the contents of some classic European specimens. Yes, you can to steal the recipe for […]
Loving this, thank you. Any help with getting the Gerolsteiner recipe minerals to dissolve?
Also… Food grade plaster of Paris? would this go under any other name?
Maybe the Gerolsteiner is a supersaturated solution. You would have to dissolve the salts in hot water. (Just wondering — I’m no chemist..)
Ian McCarthy: Try using hydroxides in stead of carbonates. If you google “food grade gypsum” you’ll find some potential sources. Brewery supply stores seems like a good starting point.
I haven’t been very successful with Gerolsteiner (my favorite water), and I’m using hydroxides. I have not tried dissolving them individually yet before combining them, nor have I tried hot water. I did make up 10L worth of solution and kept it in a 1L plastic bottle…don’t do that. By the time I got back around to using it, it had hardened/cracked the bottom of the plastic bottle and was leaking unbeknownst to me in my fridge.
Roger: Thanks for your feedback! I would not recommend making concentrated solutions as hydroxides can develop quite some heat when dissolved in water. Also, it’s important to remember that the hydroxide solutions are basic and undrinkable until they have been carbonated, so an uncarbonated solution should really be kept out of reach of children!
Regarding Gerolsteiner and dissolution I think you then should try to add the salts in the following order (as suggested by Darcy O’Neil in Fix the pumps):
1) Potassium bicarbonate, sodium chloride, sodium bicarbonate
2) Calcium nitrate
3) Magnesium sulfate, Magnesium hydroxide (or magnesium carbonate), Calcium hydroxide (or calcium carbonate)
Mix each group of salts with a little water, and the add the solutions in the given order to the rest of the water and carbonate.
If you try it, please let me know if it works!
About Gerolsteiner, and some other not easily dissolved:
have you checked final pH in the “artificial” water?
Is the same than in the natural water (not indicated in Martin’s table)?
Anybody can tell us Gerolsteiner’s pH?
Even when you make buffers, you have to adjust final pH whit HCl or NaOH. The ammount is so small than ion concentration barely change.
I quote from the Gerolsteiner homepage:
Gerolsteiner Sprudel, Gerolsteiner Stille Quelle and St. Gero Heilwasser have a pH-value of 5.9 to 6.0.
This is actually slightly more alkaline than the calculated pH of “synthetic” Gerolsteiner, so I’m not sure if this can explain the solubility problems…
It seems that Gerolsteiner is really challenging to replicate at home 🙂
I have a bunch of old formularies and beverage industry manuals from the late 19th and early 20th centuries. They have many recipes, or “receipts” as they were called then, for mineral waters. A good dozen are standards with the same formulation across different authors and decades. It’s not a new thing, but it’s nice to see an old technique revived.
If you look in old soda fountain manuals and formularies you’ll find that most of the mineral waters were made on-site. There were standard formulations for all sorts
[…] blog Khymos hаѕ done thе dirty work аnÔ ÑƒÎ¿Ï… Ñаn download hÑ–Ñ• mineral water calculator fοr Perrier, Gerolsteiner, аnÔ Vittel. Darcy […]
Thank you for doing this. I’m sure it’s incredibly helpful, but as someone who is incredibly dense, I’m having a hard time with it.
I’m trying to make this as easy as possible. My favorite water is San Pelligrino. It sounds like trying the Burton Salts would give me a close match. If not, I’d probably be willing to adapt, as I’d rather buy one product already mixed than have to find all the ingredients and combine them myself.
So, if I have Burton Salts and using your spreadsheet, I should add approx. 1.2g of the salts to make San Pell (or something close to it)? Is that correct?
Dave: Yes – Burton salts are a pretty close match, and they are avaiable from a number of sources. 1.2 grams of Burton salts for 1 L of water should be a good starting point if you use soft water (i.e. water with low calcium and magnesium levels). If your local tap water is pretty hard you should maybe lower the amount to 1 gram.
This is really fascinating work. I’ve been able to find sources for all of the salts except for Calcium Nitrate. Any suggestions on where to find this? Thanks.
[…] then… one day, someone sent me A Link. There is a whole world of water nerds out there! People who have spent time perfecting the […]
Why is the calculated pH for artificial San Pelligrino (~5.25) so much lower than for the real stuff (7.7)?
Thanks for the article, i would like to try to make my personalized mineral water.
Great stuff. I heard about you in the latest issue of wired and I’m so glad I found your site. Looking forward to trying my own “home brew” mineral water and to learning more about the science of cooking.
If I start with water filtered with a Reverse Osmosis (RO) filter, would the “composition of my tapwater” values ll be set to 0?
Pip: Yes – that would be a good approximation for RO water.
What is the saftey like with this, I want to mix Calcium Sulphate, Magnesium Sulfate and Bicarb Soda into water? Will I get some crazy by-product like sulfite from this? Don’t know much about chemistry but I dont want to creat some bad chemical reaction.
Nature does exactly that for you, deep in the ground, when water dissolves minerals and creates natural mineral water. As long as you stick with the compounds you mention you are quite safe. No crazy by-products. But as I’ve mentioned you may run into problems with solubility (i.e. that some of the salts precipitate and end up insoluble at the bottom of the flask).
Thank you for the significant amount of effort you have put into making the information of home cloning of mineral waters available.
I’m exposing the fact that my last inorganic chemistry class was 57 years ago when I ask the following: can some other acid than carbonic acid be used to clarify the solution to produce a still mineral water?
My wife drinks San Pellegrino for health reasons, but because of the carbonation, which is bad for her GERS (Gastro-Esophagial Reflux Syndrome), we pour the room temperature San P. into an open flask and allow it to de-gas prior to refrigerated storage.
My goal would be to try the Burton salts simplified recipe to approximate flat or still San P., and of course we’d like to have the taste without having to de-gas.
Thanks for your response.
Technically yes. But you asking the question suggests to me that you don’t have much chemistry background, so I wouldn’t recommend that you try!
But apart from that, if you do (on your own risk): You need an anion that will not precipitate calcium, so this excludes sulfuric acid. Instead you could use hydrochloric acid or phosphoric acid. Make sure you get food grade acids. BUT – be very careful! You should preferably know some chemistry to be able to calculate the correct amount as only very little is needed (enough to dissolve the salts, but not too much to avoid a large drop in pH). You will of course also need to know how to handle the acids safely.
May I ask – if it’s not for taste, but for health reasons – which ions are of particular interest? There should be simpler ways than messing around with all of this.
I have wondered about the possibilities of doing this at home for long while now and decided to do a little research on the subject. Your findings are very well documented, however you haven’t mentioned the one thing that made me want to research this in the first place. I really enjoy sparkling and bubbly water, however for me it is less of an issue of taste than it is an issue of bubble size and content. A home carbonator is great, they are simple and cheap and easy to maintain, however the bubble size and content is so much larger than sparkling mineral water. When you mimic these formulas, and carbonate them, do the minerals in the water cause the release of the dissolved carbon dioxide to lessen or weaken, resulting in finer bubbles?
I’m being long winded, but what i’m asking is if by mixing these minerals into the water and than using a home carbonation device, do you still get big bubbles? or are they tiny bubbles? Thanks again!
Very good question! I fired of a quick search and found a paper entitled “The effect of electrolytes on bubble coalescence in water” (requires subscription, but 1st page is free). Simply put it concludes that bubble size decreases with increasing ion concentration! So yes, you should obtain smaller bubbles if you add some extra salts to your water.
I really appreciate all the work you have done, I’m really interested in trying this myself. Unfortunately, my preference is Fiji, which includes silica SiO2. Neither Fiji, nor silica have been addressed. I’m sure I can figure out the calcium and magnesium based on the information above, but I’m curious to know if there is anything I should be aware of regarding the silica. Thanks for the inspiration.
I think this is a great idea. I’ve been addicted to sodastream water for several years now, but I agree that the taste of a good mineral water is another step up.
I ordered a mineral test for my tap water from Ward Laboratories Inc.
I spoke via email with a guy named AL who was very helpful. Their email address is:
customerrep@wardlab.com, url – http://www.wardlab.com
I ordered this test below from them (W-5). I hope it’s sufficient.
I was going to order two tests, one for my tap water and then one after it
goes through my Britta filter, but I got cheap and only ordered the post-filtered water.
After all, that’s what I will be using. The Britta removes chlorine taste and other stuff I guess,
and generally the taste of the filtered water is good, but not “tasty” like mineral water.
W-5 Household Complete Mineral Test . . . . . . . . . . . . . . . . . . . . . . . . . . .$26.50
Sodium Nitrate
Calcium Fluoride
Magnesium Iron
Potassium Electrical Conductivity
Carbonate Est. Total Dissolved Solids
Bicarbonate pH
Chloride Total Hardness
Sulfate Total Alkalinity
I hope that we can get more input about how-to’s. Like some other folks,
I’m concerned that I might create a concoction that is bad for me.
I’m hoping some scientific type will tell us exactly where to get the food
grade minerals and how to mix them up.
Thanks much for this web site !
Hi Martin,
I finally found all the minerals in your list above at Amazon in food grade, except
for calcium sulphate hemihydrate (plaster of paris) and saltpeter. I nearly got calcium sulphate hemihydrate mixed up with Gypsum (calcium sulphate dihydrate), which I could find. I got saltpeter at a different web site, but the one food grade item I cannot find
is the plaster of paris (calcium sulphate hemihydrate) in food grade. Can anyone suggest where I can get that ingredient?
CaSO4·0.5H2O = Plaster of Paris, calcium sulphate hemihydrate
MgSO4·7H2O = Epsom salt, Magnesium Sulfate heptahydrate
CaCO3 = Chalk, Calcium Carbonate
NaHCO3 = Baking soda, sodium bicabonate
NaCl = Table salt, Sodium Chloride
Mg(OH)2 = Milk of magnesia, Magnesium Hydroxide
Ca(OH)2 = Slaked lime, pickling lime, CAL, calcium hydroxide
KNO3 = saltpeter – Potassium Nitrate
I also learned that USP means food grade.
Plaster of Paris and Chalk? Making me a little nauseous thinking about that soda I downed earlier 😛
Hello again Martin,
Sorry, I had missed the discussion above about folks also looking for food grade plaster of paris like myself. I’m wondering about the Burton Salt discussion though. I thought to myself, well maybe I can at least get some of the plaster of paris using a portion of Burton Salts. On the Burton package though, it says it contains: papain, gypsum. The gypsum sort of tells me that Burton does not contain plaster of paris (calcium sulphate hemihydrate), but rather gypsum (dihydrate calcium sulfate). Since your recipes call specifically for plaster of paris, I’m wondering if these two similar compounds should be discussed as interchangeable?
Thanks for any advice.
Jeff
I got my water test results back. Since it is in ppm, I read that
1 ppm is roughly equal to 1mg/L. Is there anyone in the ether who might agree or disagree with that? Assuming it’s true, my water looks like this:
calcium 19
magnesium 8
sodium 27
potassium 3
bicarbonate 91
sulfate 2
chloride 37
nitrate .1 (1/10 mg/L)
I still cannot find Food Grade Calcium Sulfate hemihydrate. But, I guess I’ll use gypsum til I know more. I’m about ready to order some ingredients. If anyone has any words of wisdom, I’m a virtual Ferengi.
I didn’t see this in the original list of salts to get –
Calcium nitrate tetrahydrate Ca(NO3)2.4H2O, but once I plugged in my water values into the spreadsheet, the recipe for Perrier called for Calcium Nitrate Tetrahydrate which I have not obtained yet.
I see I’m going to have to brush up on my chemistry in regard to solubility / ions.
I did find Calcium Nitrate, but I don’t yet know if any of the companies selling it will sell to home addresses. I kind of doubt it after looking at the material safety data sheet on it.
Aside from the noted difficulty finding some of these chemicals/salts, it would probably be a good idea to post a more stern warning that you’d better know your chemistry when mixing some of these ingredients. I know there are already a warning or two, but this particular salt looks like it could cause serious health risks in the wrong quantities, not only ingesting but handling. That is ; if you’re able to get a hold of it at all.
Good Afternoon Martin
I have just downloaded version 5 of your excellent spreadsheet. Thanks for tracking down all this data.
Do you the maximum amount calcium bicarbonate that can be dissolved in water?
Many Thanks
Steve Willis
Malaysia
The solubility of calcium bicarbonate is 16.6 g/100 mL (20 °C) according to https://en.wikipedia.org/wiki/Calcium_bicarbonate
Good Morning Martin
We found this number too. Sadly, this appears to be incorrect and is more like 1.6g per litre.
I’m sorry that my reply was a little short and simple. The solubility of Ca(HCO3)2 is a bit complex because it can’t be isolated as a salt. You need to generate it in the water by addition of CO2. There’s an explanation here:
https://chemistry.stackexchange.com/questions/118786/understanding-the-solubility-of-cahco32
As an example, in the recipe for San Pellegrino water there is a lot of Ca2SO4 that goes into solution upon bubling CO2 through the water.
Does this agree with the data you have collected on mineral waters?
Thanks for this Martin — I am a big fan and kind of obsessed with making different waters with your sheet. I am trying contrex now because I am am a “go big or go home” kind of gal and I am looking for more calicum in my diet. So far I haven’t been able to get the contrex solution to dissolve completely. Any harm in experimenting with reducing some of the ingredients to make my own version that dissolves?
@Gabriella: Thank you for the feedback! Solubility of the calcium salts is a known issue, so I think it’s a good idea to reduce some of the salts to see if that works better with your setup.
Martin,
Fantastic. I also happen to be a chemist (originally – graduated nearly 50 years ago! and worked in development, mainly in Africa – but continued to dabble in chemistry. I just came across your spreadsheets for making mineral water, really great, and tried one already. I could not get the latest 2015 excel spreadsheet to download, so will try again, but was using the 2012 spreadsheete – with recipe for Perrier. A couple of questions – impressions – and excuse me if these are already answered on your blog above, I plan to go through it now. Q1. Why add Calcium nitrate deliberately to drinking water? I thought it was a good idea to keep food nitrate intake as low as possible – unless a bit of nitrate gives certain water recipes a real added something, I will leave it out (how to know though!), Q2. Why are there often several different sources of anions, eg Calcium? surely the tase of the final water depends on the total concentration of any particular free anion?, so why use Calcium carbonate as well as Calcium Chloride? surely just calcium chloride would do and is more soluble than the carbonate – but do you think that would just put the Cl- cation concentration too high? Q3. Solubility problems aside, I will be aiming to produce a solution of ‘concentrate’ that I can add to my carbonater water bottles with a pipette, I suspect even the calcium and magnesium can be a fine suspension stable enough when shaken up before dispensing? Q4 – in following through your recipe, albeit most probably an out of date one, you referred to adding water to bottle, adding salts, then gassing up with CO2 then cooling. I would have thought it would be better the other way round, cool the water to be used first, add the salts (although less soluble when cooler) then add the CO2, am I right in thinking that CO2 is much more soluble in water at say 10 degs C than 20 degs C?
I dont know if you have written about this, but there must be statistics on the huge volume of road and sea freight, especially in Europe, dedicated to moving sparkling mineral water from one country to another, adding thousands of tons of carbon dioxide to the atmosphere in the process. Of course there is one particular multinational company that is busy shipping Italian water to France and French Water to Italy and making a very nice profit out of the process, whilst the Swiss blow up mountains to allow the companies lorries easy access to their ‘markets’. A nice sachet of your salts and a carbonating machine could help put an end to that nonsense!
Thanks David! I’ll have to troubleshoot the download issues – otherwise I will email it too you. To your questions:
1) I agree that you should be careful with the amount of nitrates. But I seem to recall that some sources have attributed a certain sweetness to nitrates – that’s why it’s included in case someone wants to investigate.
2) Certainly it’s the total amount that counts, but since anions and cations can’t be added individually you will need at least two sources so that they can be adjusted independently of each other. As you point out – the Cl- would be too high. Keep in mind that the calculator is supposed to work with a very broad ranges of mineral waters.
3) You could give it a try, but solubility will be an issue. I’m not quite sure how you intent to sample slurries in a homogenous way.
4) You are quite right that the solubility of CO2 increases with decreasing temperature. The order of addition that you describe could be a way to achieve that.
I agree that mineral water, especially if transported around the world has a significant carbon footprint. And yes, DIY mineral water could be a way around this issue! I have not plans pursing this as a business, but perhaps someone else has?
Is there any issue with using the calculator to make still (non bubbling) mineral waters? Will they dissolve just the same and taste the same?
Also is there a reason the spreadsheet lists all the ingredients in the carbonate form -Magnesium carbonate, calcium carbonate, sodium bicarbonate??
Depending on the salts you may run into solubility problems if you only add salts to still water.
I’m not quite sure I understand the second question, because in the spreadsheet you can chose between different salts. For magnesium you have the choice of magnesium chloride, magnesium carbonate, magnesium sulfate and magnesium hydroxide.
Hi, the link to v6 of the spreadsheet seems to be non-functional; attempting to download has no effect. Thanks for doing the work and sharing this info! I’m looking forward to trying some!
To those with download issue – at least for me, Chrome seems to think the link isn’t safe. Right clink -> save link as, save, then hit the little up arrow and “keep” – this bypasses the problem.
This is an awesome resource, super interested in trying this!. Just curious – 20 minutes is a long time to wait every time I want sparkling water! Will shaking the mixture help speed that up, or will that release too much carbonation?
Also, if I can’t find food-grade plaster of paris, any suggestions for aiming for a roughly equivalent recipe? Would compensating with more epsom salt be likely to still taste good?
Thanks for the work-around with the download! I must admit I have no clue how to fix this.
Glad you have found the calculator useful. Different salts take different amounts of time to dissolve, so it’s hard to be very specific regarding the time. If the water is all “white” and opaque you’ll need to wait a little longer. If the water is clear and everything seems to be dissolved of course you do not need to wait. And yes – agitation/stirring can speed up dissolution! As long as you keep the bottle capped shaking alone will not cause you to loose any carbonation.
Stores with brewery supplies tend to be a good place to look for food grade water correction salts.
Wow, I’m SO glad I found this. Thanks for all the work. For a while I’ve been making my own coffee-brewing water starting with distilled water and adding minerals. These charts will help me understand whatever it is I’m doing (LOL), and also assist in my new adventure of creating “custom” drinking water (that I may actually like).
I just noticed you have several posts on coffee brewing. Guess I’ll be perusing that stuff in the very near future. Here go another couple of hours at the computer …
Looks like the v6 link is busted. Any chance we can get a new one? Thanks.
Nevermind. I see the fix in the comment right above. Cheers!
Can anyone recommend a source for calcium nitrate tetrahydrate?
I have searched but cannot find any.
Thank you for all the great information.
It can be hard (impossible?) to find a food grade quality of this. Nitrate is the least important anion to include, so if you don’t fint it – just skip it!
For those who are wondering about replacing plaster of paris (CaSO4·0.5H2O / calcium sulfate hemihydrate) with gypsum (CaSO4·0.5H2O / calcium sulfate dihydrate), it is very easy to do!
To use gypsum instead, multiple the calculator’s CaSO4·0.5H2O amount by 1.186.
Explanation: They both contribute the same number of calcium and sulfate ions (for each molecular unit), but due to the extra 1.5 H2Os in gypsum, they have different molecular weights. Gypsum is ~172 g/mol and plaster of paris is ~145 g/mol, so you’ll need slightly more gypsum by weight to contribute an equivalent amount of calcium and sulfate.
Slight typo: gypsum is CaSO4·2H2O, not CaSO4·0.5H2O.
From everything ive read, the nitrate is used in the uptake of minerals. I dont know if thats based on flavor or absorption. Just something ive read about.
Has anyone had any luck getting calcium hydroxide/carbonate to convert to calcium bicarbonate better? I’m trying to make a high mag/calcium bicarbonate mineral water, but have a hard time getting the calcium to convert. Any tips/tricks you have figured out?
I have an excellent espresso machine, but the water after standard filtering isn’t very good. If I want to use your amazing calculator to change the minerals in the water for the coffee, can I achieve this by adding the ingredients to a water conditioning tank? Would the passthrough of water be enough to get the results for the artificial mineral water?
@Matteo: Depending on which salts you intend to use there may be issues with solubility, meaning that the salts simply will not dissolve or dissolve very slowly.