Practical molecular gastronomy, part 3
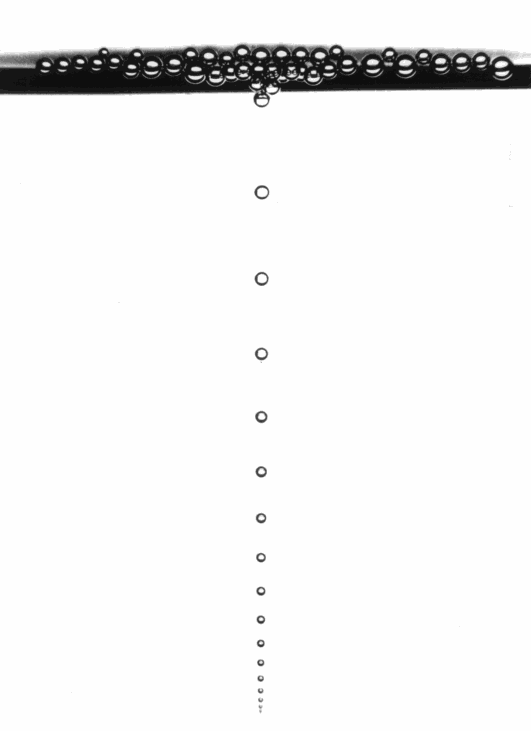
Get a basic understanding of heat transfer, heat capacity and heat conductance. Since a lot of cooking involves temperature manipulations, it’s a good idea to get a basic understandning of how heat is transferred and how well it is stored…