Get a basic understanding of heat transfer, heat capacity and heat conductance.
Since a lot of cooking involves temperature manipulations, it’s a good idea to get a basic understandning of how heat is transferred and how well it is stored in different materials. “Heat” in this context does not imply high temperature since it also applies to the understanding of freezing/thawing.
Closeup of ceramic stove top
Heat transfer
Conduction: flow of heat through an object or between two objects in contact. Metals are typically good conducters whereas air is a poor heat conductor.
Convection: heat transfer occurs because particles are moved from a warm region to a colder one. One can say that convection is a combination of conduction and mixing. For example, convection occurs when heating water since its density varies with temperature – warm water is lighter than cold water and will float. This video illustrates convection currents in water as a crystal of potassium permanganate dissolves (this salt is not edible).
Radiation: in the kitchen we encounter two types of heat transfer by radiation corresponding to two different parts of the electromagnetic spectrum. The heat we feel from hot burning charcoal, a stove top or the sun are all a result of infrared radiation. The other type is microwave radiation. Heat transfer by radiation does not require a material for the heat to pass through (as a consequence, a blowing wind will not have any significant effect when grilling). Microwaves easily penetrate plastic, glass and wood, but not metal. Infrared radiation is blocked by opaque materials.
Heat capacity and heat conductance
Heat capacity: the heat requried to raise the temperature of the material. Water has a very high heat capacity, metals (shown in red) generally a low heat capacity.
Heat conductance: how well heat flows through the material. Some metals (shown in red in the graph) are excellent heat conductors (silver, copper, aluminum), others less so (iron and stainless steel). All other materials (shown in blue) are generellay poor heat conductors.
The heat capacity (or to be precise, the specific heat capacity – which means heat capacity per weight unit) and the heat conductance of materials encountered in the kitchen are plotted in the the graph below:
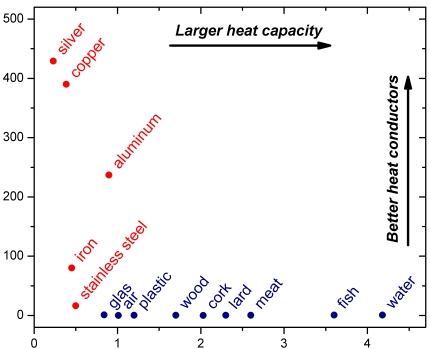
(for the technically interested, the plot units are Wm-1K-1 for the heat conductance and Jg-1K-1 for the specific heat capacity)
For a more extensive treatment of heat transfer, heat capacity and heat conductance (+ more on cooking methods and materials) in a gastronomical setting, I recommend the Gourmet Engineering Lecture Notes for a very interesting course given at Tufts University in Medford, MA, USA. Cooking for Engineers also has a nice post on heat transfer and browning of foods and one on common materials of cookware (with comprehensive comparisons of different materials used).
Examples related to food preparation and handling
Convection ovens utilize fans to circulate hot air allowing reduced cooking times and temperatures. Because of efficient convection, two or more trays can be baked simultaneously.
In a steam oven water is introduced to increase the humidity (this can also be done by spraying water into the hot oven). Heat transfer is more efficient due to 1) the higher heat capacity of humid air and 2) the energy released when steam condenses onto the surface (it’s the energy it took to boil the water in the first place). For bread, the condesed water prevents the surface from drying out which facilitates the exapansion of the loaf. Furthermore, the hot surface causes starch to gelatinize and subsequently dry into a delicate crust.
Water will cool faster than the same volume of a thickened soup because of less resistance to the convection currents in water. The amount of convection decreases in the following order: water > chicken soup > creamy soup > thick onion soup > porridge. In the latter heat is transferred by conduction only from the interior to the exterior (where heat transfer proceeds mainly by radiation and conduction). This will also affect cooling times, which is of importance with regard to microbial safety (food should be cooled rapidly past the window from 30-60 °C where microorganism thrive).
For rapid defrosting, place the frozen food in cold water or on a metal object – this will allow an efficient transport of heat to the frozen food. Defrosting in a microwave is not easy because most of the water molecules are locked in rigid structure and even microwaves cannot make them move (they only melt by conduction of heat from melted neighbouring areas).
To freeze icecream or a parfait, use a metal container as this will allow a faster dissipation of the heat in the freezer.
When whipping cream, it’s essential to keep the temperature low (otherwise the fat will melt). Use a thick glas bowl and cool it in the freezer before whipping.
When cooking meat in a pan or on a grill, notice how the surface browns relatively fast compared to the time it takes for the interioir of the meat to heat up. Heat transfer to the surface by radiation or conduction is very efficient compared to conduction of heat through meat itself. Therefore it’s advisable to fry/grill the meat at high temperature first to get a nice browning, then let the meat rest for 5-10 min to allow for heat conduction to the interioir (cover with aluminum foil to reduce radiative heat loss), followed by a second frying/grilling at lower temperature until desired doneness.
In an oven, the heating caused by radiation can be increased by moving food closer to the walls or reduced by wrapping the food with reflective aluminum foil. For example, to caramellize sugar on a creme brulee if you don’t have gas burner, place them as high as possible in the oven, preferably using a grill element. Turkey legs stick out and easily get overdone – wrapping them with aluminum foil reduces heat radiation from the oven walls.
For a bain marie, always use a metal bowl as this gives you better temperature control. When making egg based sauces such as hollandaise or bernaise, use a thin metal bowl this allows rapid heating and cooling (if temperature gets to high, the metal bowl allows quick cooling which might save the sauce).
A pizza baking stone has a higher heat capacity than a metal plate/sheet – this ensures proper rising and gives a crispy crust.
Ever burnt your tongue on a pizza? Tomatoes (mostly water) retain heat far better than the crust (many air bubles, low heat capacity) and cheese topping (cools fast due to radiation from surface).
The vacuum in a thermos does not conduct heat by conduction or convection, only by radiation. The latter is minimized (in thermoses of glas) by a silver or aluminmum coating, creating a reflective mirror.
From the graph it doesn’t seem like cork is a particularly good insulator. This is because the heat conductance is plotted per weight unit. For a porous material such as cork, the effective heat conductance is much lower than for the same volume of other materials.
Lastly, just to illustrate how complex heat transfer and convection sometimes can be, take a look at the Mpemba effect: Believe it or not, under certain conditions, hot water freezes faster than cold water!
*
Check out my previous blogpost for an overview of the tips for practical molecular gastronomy. The collection of books (favorite, molecular gastronomy, aroma/taste, reference/technique, food chemistry) and links (webresources, people/chefs/blogs, institutions, articles, audio/video) at khymos.org might also be of interest.



Perfect! Let me ask you a question (´cause we were talking about this at work today)
Say that I put food in a sealed vacuum bag in two different ovens. One with dry heat and the other with 100% humidity. Same temperature. Would the oven with steam cook the food faster? The end result would be reached first in the humid oven?
/ john
Yes! The moist air will accelerate the heat transfer. Think of it this way: As long as the surface of the vacuum bag is below 100 °C, moisture from the air will condense on the surface. In this process heat is transferred from the water molecules to the surface of the bag.