
Egg cooked for 40 min at 63.0 °C. The pictures were taken within 6 seconds and are shown in the order they were taken.
My immersion circulator is working again! And the first thing I decided to do was to cook eggs at 63.0 °C for 40, 60, 75, 110 and 155 min and show you the results. If you read my last blog post on Perfect egg yolks or have stumbled across the paper Culinary Biophysics: on the Nature of the 6X°C Egg you may recognize that these times correspond to egg yolks with textures similar to sweetened condensed milk, mayonnaise, honey, cookie icing and Marmite respectively. I used the iso-viscosity graph from the paper mentioned to determine the cooking times as shown below.
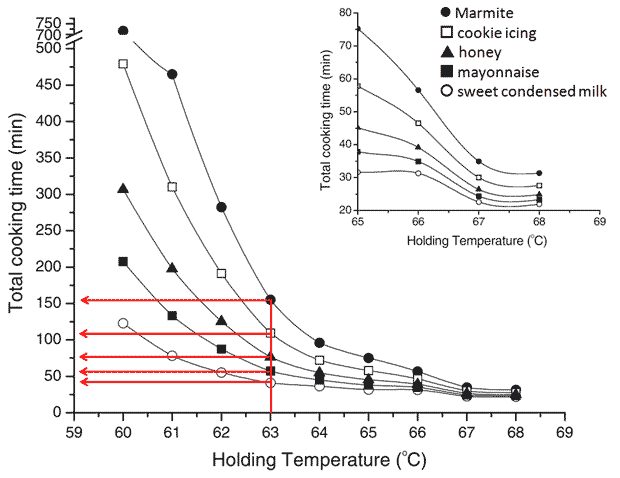
The figure shows how cooking times at 63.0 °C are determined to achieve different textures. (The figure is used with kind permission from Springer Science+Business Media: César Vega and Ruben Mercadé-Prieto in Food Biophysics 2011, 6:152-159, Culinary Biophysics: on the Nature of the 6X °C Egg, figure 8, page 158. The legend overlay has been added by me for clarity.)
As the individual eggs reached their cooking times they were held in cold water until the last egg was finished. I then cracked all the eggs and took the pictures below to illustrate the differences in textures. I think the picture speaks for itself. The amazing thing is that the only difference between the eggs is the cooking time!
It can be difficult to judge textures properly from still photos, so I also shot a few video clips to illustrate the texture of the 40, 75 and 155 min eggs (by the time I shot the videos the yolks had become more viscous, possibly due to cooling and/or evaporation). The texture ofthe 155 min egg yolk was perhaps the most fascinating with a tremendous plasticity. There must be some exciting culinary uses for this!
If an egg is to be served by itself one will typically also want to set the white. There was a question about this to my previous post, and a reader even tried with 2 min pre- or post-boil. Without cooling the difference between pre- and post-boil was quite significant as evidenced from the pictures. I did a similar experiment but cooked the eggs at 63.0 °C and opted for a 3 min pre- or post-boil with the small difference that I cooled the egg back to room temperature after/prior to the pre-/post-boil to avoid any interference between the 63 °C and 100 °C treatments. This worked very well and I wasn’t able to detect any difference between the pre- and post-boiled eggs.
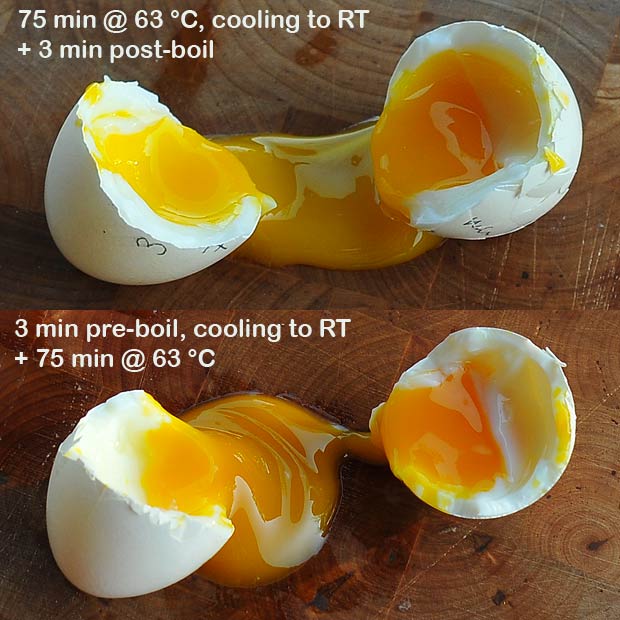
It doesn’t matter if you pre- or post-boil your egg as long as you cool it to room temperature inbetween the boiling water and the temperature controlled water bath.


[…] and then boil the egg for 3 min to set the egg white (also can pre-boil egg instead). Link Share […]
Two days ago I sent an email to remind myself to mention this to you: I realized that we haven’t discussed the effect of the serving temperature on the texture of the egg. For instance, when I cook duck yolks at 64.5C or chicken yolks at 64.8C to use as a pipe-able sauce (http://www.eatfoo.com/archives/2011/04/duck_fat_crackers_duck_yolk_bl.php), they are still fairly runny while warm, but set up much harder once chilled. If re-warmed, they become less viscous again. So, it’s pretty clear to me that the cooking temperature and time are not the only factors: the serving temperature matters as well. If you crack the eggs and serve straight from the bath, you’re going to get a different texture than if you serve 5 or 10 minutes after removing from the bath.
Because I typically do dinners of around 20-30 diners, and plate up all the plates at once, doing soft-cooked eggs has always been somewhat inconsistent. The best process I’ve found for serving is as follows: remove all the eggs from the bath, crack the shells of all of them by tapping with the back of a knife (but leaving the eggs inside the now-cracked shells), then prying open the eggs at the cracks and dumping the contents of each egg out onto a slotted spoon and then onto the plate before moving on to the next egg. This process takes about 5-10 minutes from start to finish. I typically have one or two people in the kitchen working on this while others are plating the other components of the dish.
I have definitely noticed that the texture of the eggs is more viscous by the time the diners break into them that it is in the tests I run for temp/time beforehand. The texture is still good, but it’s different, and so I have had to compensate for it. I prefer a 63.5C yolk when cracked and eaten immediately, but I do my eggs at 63C now for service to compensate for the delay.
Barzelay: The viscosities in the paper were measured at 25 °C. It makes sense that the viscosity increases as the egg yolks cool. But given the importance of time and temperature – what do you mean when you talk about 63.0 and 63.5 °C? (i.e. what cooking times are you using)
fooducation: I notice that with >45 min at 68 °C Anu’s eggs fall outside the plots of the Vega’s paper. But the idea of cooking them separately is nice though 🙂 Regarding your comment about surprise – I think the reason is simply that even scientists (Hervé This as well as others) have focused so much on temperature that we have ignored the effect of time. Many may have had inklings that there was an extra variable, but no one was able to piece this together in a systematic way. Thinking about it in a strictly chemical way however it makes sense that the denaturation is a kinetic phenomena where both time and temperature matter.
My experience is also that the yolk sets upon storage at room temperature, which is not surprising due to Brownian motion phenomena (but there might of course be more to it than this). An application that works fine, taken from from Anu Hopia’s blog, is as follows:
– 6X °C-cook the eggs as desired
– crack the egg, pick out the yolk, rinse the yolk for white and leave
– fry the white separately, I used a buttered muffin tray in the oven until set
– garnish: the white at the bottom sprinkled with salt, pepper and chives. The “yolk-ball” on top sprinkled with Maldon salt and pepper
being an amateur cook, I had no great difficulties serving this to 10 persons at home as a slightly warm starter together with gazpacho (“how to make tomato soup with boiled eggs the difficult way”-sort of thing).
Pictures and ref. to Anu’s post at http://www.fooducation.org/2011/02/6x-c-egg-or-opposite-boiled-eggs.html
Finally, I’ve reflected upon that we/people are surprised that setting of the yolk is a result of both time and temperature. This should really not be a surprise, should it? Colour development etc. in meat is mainly a result of temperature and not time, but several other processes are time dependent, right? These are rather complex systems, anyhow…
Pretty impressive post. Now, if only the water bath circulators weren’t so expensive…
Thank you for showing the video and for the white-setting protocol. They were really helpful.
Isaia: you really don’t need a circulator bath, as long as you’ve got a decent kitchen thermometer and a large pan with a lid. Since water has such a high heat capacity, what you need to do is use ridiculously much water compared to eggs. Keep the hotplate on minimum/low, and you’re pretty much there. See the blog post I refer to above for a description.
Perfect? Perfectly underdone. Perfectly unpalatable.
Soft-cooked eggs are an abomination in the eyes of the Lord
BTW (sorry for repeated comments): the age of the eggs should definitely make a difference? Protein degradation over time in fresh (and less fresh) eggs results in the white becoming more fluid. After all, egg albumen height, a direct result of albumen viscosity, is used as a freshness indicator.
My experience is, indeed, that the egg whites behave quite differently when I’ve cooked eggs at 6X °C. Last time, I used a mix of older and fresher eggs and noticed a marked difference among the whites but didn’t think of looking closer at the yolks…
So, do we have information on how the yolk texture/proteins develops during storage? (compared to the white which seems to be fairly well researched)
fooducation: The floor is yours 🙂 Buy some fresh eggs now and a couple more in a few weeks and compare them systematically. I also seem to remember that the pH of the white will rise with time.
Barzelay: I think the cooking times are cumulative, but this actually remains to be checked. Maybe Cesar knows?
“what cooking times are you using?”
Well, like most, I wasn’t paying much attention to the cooking times. I would cook the eggs at 63 (or 63.5) for maybe 45 to 90 minutes, then chill them rapidly and hold them for up to 3 days or so in the refrigerator, then hold them for up to 90 ADDITIONAL minutes at 60C during service.
Here’s another question: are the times cumulative if the eggs are held at a particular temperature, cooled, then held at that temperature again?
I think a pervasive pitfall of many scientifically-interested cooks is the conflation of thermal equilibrium and thermodynamic equilibrium. The cooking of egg yolks is a great illustration of the problems that can arise. Though the temperature is homogenous, 63C let’s say, the kinetics of gelation are still limited by time. It’s curious that cooks do understand that short ribs or a piece of chuck roast change in texture over dozens of hours in a fixed-temperature waterbath but never made the connection to eggs. Perhaps it is because egg yolk is so seemingly simple and standardized that curious minds overlooked them. Thanks for spreading the word! Thanks too to Fooducation, who it looks like you may have heard of the study from.
After reading the Vega & Mercade-Prieto paper, I was most intrigued that similar viscosities could be evoked from eggs at different temperatures. Since they only characterized the viscosity at a single shear rate, I was curious how the actual mouthfeel of the yolks would compare. There is a lot more going on in the mouth than can be accounted for in that one data point, though the researchers do admit that psuedoplasticity, which they did not account for, plays a significant role in texture perception. I tried a few time & temperature combos from the iso-viscosity graph shooting for the “cake icing” texture. One variable I was able to eliminate was the source of the eggs, since Columbia, Maryland is just a few minutes from my house, haha. Neither you Norwegians nor that San Franciscan can replicate THAT one. I hope to put up the results of a tasting-panel evaluation over the weekend. I can tell you now that, as a former believer in the “63C egg” I’m pretty blown away by the results. Subtle differences, but even the whites are incredibly similar.
And Barzelay brings up an excellent point. I hadn’t really paid attention to serving temperature. I had assumed, again incorrectly, that once the egg was gelled and then set at a cool temp, the texture was fairly constant. Instead it appears to be more like a caramel. The texture is defined by the temperature that the initial ingredients are heated to, which defines extent of caramelization, but also by the serving temperature. This makes me wonder if caramelization is also a kinetically-limited process. It is possible that at the higher temperatures required for caramelizing the carmelization kinetics are faster than the diffusion of heat through the solution, in which case they can essentially be ignored.
When making caramels, or any other hot sugar candy, I had always assumed that heating rate was unimportant. Go slow enough that you don’t burn it and the temperature of the boiling solution will indicate the relative sugar/water content. I guess the problem with a boiling sugar/water system is that you can’t dwell at a fixed temperature since water loss will cause the solution’s boiling point to increase. Maybe you could dwell at the final temperature using a controlled heat source so the temperature stays fixed, despite water loss, but that would be more difficult. Also it would be difficult to deconvolute the effect of the additional water loss from the effects of additional dwell time on caramelization kinetics. Have you heard of any chefs/scientists/enthusiasts that alter their sugar candy textures/properties by going to a particular final temperature at different heating rates, or by dwelling the solution at final, controlled temperature?
Colin: A thorough comment – thanks! I actually stumbled across the paper via google searches, but of course I also know Erik’s post at Fooducation – he’s a former colleague of mine and I read his blog regularily.
Regarding the kinetics of caramelization there are actually quite a few publications on this. See for instance this book or these papers. Even at lower temperatures you can observe caramelization, especially at high pH.
[…] Perfect Egg Yolks (via Khymos) This guy has creating the perfect soft-boiled egg yolk down to a science. Make sure to read both Part 1 and Part 2. […]
The next question, I suppose, is how to handle egg yolks separately. If you solely put yolk in the bag and thus don’t have to use a temp high enough to cook yolk/penetrate, can you control texture by temp alone? I assume not on the basis that it depends on the extent of the reaction, but it would be good to test.
[…] 63°C eggs. This graph about cooking temperatures and times for desired yolk consistency from this website was THE most helpful. If you are interested in experimenting too, it is a very through examination […]