Here’s some pictures of an experiment I did with strawberries and dry ice (solid carbon dioxide). Dry ice is frozen carbon dioxide which holds a temperature of -78 °C. What is fascinating is that dry ice does not melt – it sublimes, which means that it turns directly into carbon dioxide gas.
The idea was to create a carbonated fruit which gives a sparkling sensation in the mouth. I have used strawberries, but any juicy fruit with a moist surface could be used. Water melons would be perfect!
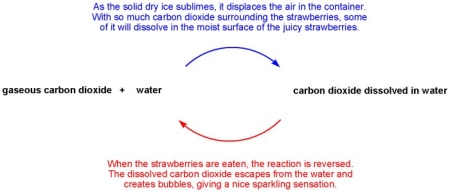
The chemistry explained in simple terms:
A schematic drawing of the container:
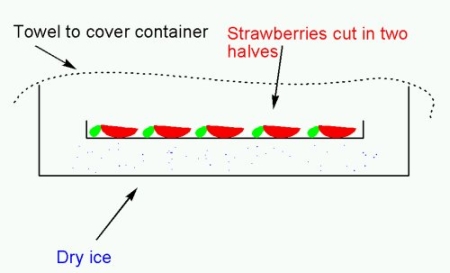
To prevent the plate from touching the dry ice (which would cause the strawberries to freeze), I put in a wooden triangle first.
Put the plate with strawberry halves on top of the wooden triangle. Cover with a kitchen towel (do NOT cover with a tight fitting cover – remember that as CO2 sublimes, it expands, and this would create a huge pressure ultimately resulting in an explosion), and leave for 30 minutes.
Eat and enjoy!
Update: Carbonated fruit the iSi way!

Just a thought, if you have a pressure cooker then you probably can get a hell of a lot more CO2 in your treats and you don´t have to be worried about an explosion.
I just made these and they’re awesome! Thanks!!
I tried this with orange slices (I’ll try in spring with strawberries) and it didn’t work very well, even with a cap that made a little pressure. I think this happened because in oranges water is closed in those little “drop-shaped bags” (sorry but I don’t know the correct word in English). I think I will try again with another juicy fruit, or I’ll wait for strawberries to grow in my garden…
Mo: Assuming you sliced the orange so as to expose the interior of the juice sacs to CO2, I can think of another possible explanation. At low pH, the following equillibrium will be shifted to the right:
CO2 + H2O ⇔ H2CO3
This means less CO2 will dissolve in the juices. I guess the pH of oranges is significantly lower than of strawberries. Have you tried it watermelon instead?
I should try with watermelon, but I’ll have to wait some months, this is not the right season.
Hi, sorry if this is a silly question, but how/where would you get hold of dry ice to do this? It sounds like fun!
In Shanghai the Hagan Daz gives you dry ice when you buy an ice cream cake. I tried it with the strawberries and it’s great with the ice cream cake. Shanghai’s got strawberries allyear round, greenhouses all over Chian now.
Jessica, some grocery stores in the US sell dry ice.
When I was in elementary school my dad used to get dry ice for our volcanos from Baskin Robins, if you just go in and ask nicely I think they’ll help 🙂
[…] blogged about carbonated strawberries some while ago. Those were made using dry ice which unfortunately is not always easy to get hold […]
A question, what is the difference between leaving the strawberries in a saturated CO2 atmosphere using dry ice or CO2 gas?
If you do not freeze you cannot oversaturate the strawberries with CO2 under ambient pressure? Or am I mistaken.
Will try it nevertheless, enough dry ice (and liquid N2…) in our lab.
Jurgen,
At room temperature dry ice sublimes, meaning that the solid transforms directly to gas without melting to a liquid first. So if the container is filled with dry ice it will slowly displace the air as it sublimes, leavning the atmosphere saturated with CO2.
The amount of CO2 that will dissolve in the strawberries will be dependent on the temperature, pressure and concentration of CO2. The experiment is performed at ambient pressure, a temperature close to 0 °C and in pure CO2 gas. The only way to get more CO2 to dissolve is to increase the pressure. The safest way to do this is to use a iSi container which is designed for high pressures (blogpost). Do NOT pressurize a container using dry ice!
Thanks. I guess I was surprised that you could saturate a strawberry with CO2 by just placing it in a CO2 atmosphere.
I tried carbonating fruit by filling a 2 liter coke bottle with fruit and dry ice. How big can I get the bubbles to be, and do you have any times on getting them bigger?
Aaron, I wouldn’t recommend using a (closed) bottle for this. When dry ice goes from solid to gas it’s volume increases dramatically leading to large pressures – and possibly an explosion…
If you wan’t to make carbonated fruit, check out this blog post where a iSi whipper is used:
http://blog.khymos.org/2007/04/09/carbonated-fruit-the-isi-way/
Regarding bubble size – I don’t know if I understand your question correctly. Why do you wan’t larger bubbles?
Well actually I was following the instructions on the site
http://www.instructables.com/id/How-to-Make-Carbonated-Fruit/
I think their method would work decently as the bottles can withstand decent amounts of pressure and are cheap to obtain.
I was actually planning on doing this as an activity for a club, so I have to keep the cost down. I mostly want larger bubbles so it will have more of the carbonated effect. Any smaller would be kind of a letdown, because most people would expect relatively large bubbles. How big are the bubbles with this low-pressure technique here? Currently, the bubbles are so small that they end up as just a very mild tingling rather than any popping.
Personally I wouldn’t dare to use plastic bottles. Unless you’re able to do the calculations required to determine a safe amount of dry ice, this experiment can take a really, really bad turn.
The iSi whippers however are safe to use with the designated soda chargers (which contain carbon dioxide). I don’t know what your budget is, but you can get whippers for less than $50.
I think what you refer to with “larger bubbles” is to dissolve more carbon dioxide in the fruit juices (based on what you write about “small bubbles” which are barely noticeable). To achieve this you can cool the bootle to right above freezing as the solubility of carbon dioxide increases with decreasing temperature and leave it there pressurized for some hours. Futhermore you can increase the pressure by adding more dry ice – which of course can be dangerous if you use plastic bottles…
how long does the carbonation last or does the fruit go flat lol or is there a way to preserve it??? thanks aND GREat blog btw!!!
It is simple to carbonate fruit using a co2 dispenser (under $20 at most bike shops), food grade co2 cartridge, Fizz-Giz bottle cap and plastic drink bottle. You can do it safely with a regulated co2 dispenser. Set your injector pressure to under 60PSI. 3-liter bottles have larger mouths than smaller PET bottles. That makes it easier to get your fruit in (and out). You can get caps at http://www.FizzGiz.com and co2 injectors at Genuine Innovations in Tucson, AZ. Be sure to ask for the HOT sprayers.
I tried this. How much dry ice does it take to slowly dissolve in 30 minutes? Do you add water (warm, hot, room temp or cold) I failed totally
Joe – co2 gas must be forced under pressure into ‘stuff’ you wish to carbonate. A lot of web advice seems to come from people who haven’t a clue. Although you can submerge ‘stuff’ under a bath of co2 gas, it doesn’t carbonate ‘stuff’ any more than co2 from a fire extinguisher carbonates a fire.
Unless you force co2 under pressure into ‘stuff’, your ‘stuff’ won’t get carbonated. A gentle breeze of co2 gas blowing over a strawberry will no more carbonate the strawberry than a gentle breeze of air blowing over the strawberry will aerate it.
The guy that suggested a pressure cooker wasn’t too far off the mark. The weight on top of the older model pressure cookers will, best as I recall, get you an additional 15PSI inside most cookers. That’s about 30PSI, which is enough for beer & strawberries. Another issue you should prepare yourself for is a long wait to infuse co2 gas into your “stuff”. At lower pressures (like 30PSI) – even at 55 to 60PSI, be prepared for a day-long wait – maybe more.
Mike: If you read my blog post carefully, you’ll realize that I actually achieved carbonation without using pressure! When the strawberries are cooled in an atmosphere that is saturated with CO2 you do get a decent amount of carbonation.
If you want to use pressurized CO2 you should be really careful about which container you use. Only use a container that’s made for this purpose and equipped with appropriate safety vents, otherwise you can have a serious explosion.
I’ve done pressurized carbonation with a iSi whipper – read more about that in the blog post: Carbonated fruit the iSi way
Am planning on using the co2 rich environment. However, how many pounds of ice for 8 strawberries. 1/2 hour is what I’ve read various places. Pour in say 1/4 cup warm water, listen for a while then pour in another 1/4 cup warm water. Do this for 1/2. Then how long will the carbonation last. I assume you place in refrigerator during the waiting period to serve
Have you any ideas?
Joe: 1-2 pounds should be plenty. But don’t pour water onto the dry ice! What happens then is that the water get’s carbonated (rather than your strawberries). When exposed to ambient temperature the dry ice will convert to CO2 gas just by itself. The carbonation will only last for a couple of minutes, so don’t remove from the CO2 atmosphere until you’re ready to serve.
Thanks Martin. Will fill you in. Also doing cotton candy fois gras, whipped chocolate mousse and spherified mango over watermelon
Try it out, Joe. When you’re done, drop one of your strawberries into a martini glass of chilled water and let us all know if you see bubbles of co2 gas arising off the surface of the berry, floating to the surface. And let us know if you get a distinct taste sensation from the co2 infused berries. Once you know, let us all know. Martin says it worked for him. I look forward to reading of your own experience.
I can say with confidence that white grapes maintained under pressure at 60PSI in a pure co2 environment inside a 3-liter bottle for a day pick up enough carbonation to emit a steady flow of bubbles when immersed in water in crystal wine glass. To look at it, one would think they were bathing in champaign.
I would imagine that the water would do little more than speed the process along. The solubility of CO2 in water is greatly reduced at higher temperatures. I believe that only a marginal amount of CO2 would dissolve in the water. The water would most likely just cause the dry ice to sublime quicker, releasing more CO2 into the container.
I’ll add this to the recipe. Will attempt again on Saturday and report back
Rob, you’re right. If all U R doing is bathing the berries in a co2 gas at atmospheric pressure, why waste the co2 by evaporating it all in 1/3 the time by surrounding it with warm water. Just throw the dry ice and the berries in a box & wait.
Hopefully Joe will try it and let us all know the results.
Hi there Martin!
I did this experiment today and it worked well! However, I was wondering, can you do the same to raisins after plumping them and cutting them in half? I tried doing that today but it just froze instead of becoming carbonated. Also, I did it with grapes as well, and it froze too. I checked if my bowl was touching the dry ice but it wasn’t. What do you think could be the problem?
Hey Joe. Been 17-days. Thought I’d check back on that report you promised us. Care to comment? Lookin’ forward 2 it.
Thanks for the followup. I think tomorrow or Sunday. Will try no H20 and let them stew undercover testing throughout the day. Haven’t forgotten u’all
We carbonate pomelo successfully in vac pac bags